Scientists have moved a step closer to the goal of creating stem cells perfectly matched to a patient's DNA in order to treat diseases, they announced on Thursday, creating patient-specific cell lines out of the skin cells of two adult men.
Scientists have moved a step closer to the goal of creating stem cells perfectly matched to a patient's DNA in order to treat diseases, they announced on Thursday, creating patient-specific cell lines out of the skin cells of two adult men.
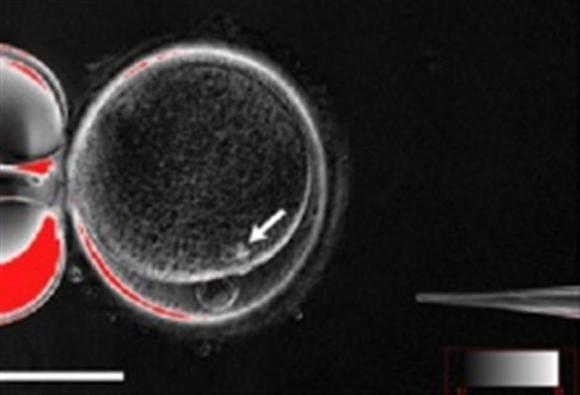 |
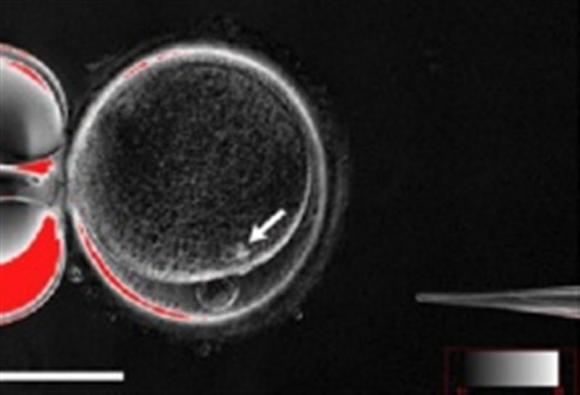
| A handout picture shows the process by which scientists created patient-specific stem cell lines out of the skin cells of two adult men. |
The advance, described online in the journal Cell Stem Cell, is the first time researchers have achieved "therapeutic cloning" of adults. Technically called somatic-cell nuclear transfer, therapeutic cloning means producing embryonic cells genetically identical to a donor, usually for the purpose of using those cells to treat disease.
But nuclear transfer is also the first step in reproductive cloning, or producing a genetic duplicate of someone - a technique that has sparked controversy since the 1997 announcement that it was used to create Dolly, the clone of a ewe. In 2005, the United Nations called on countries to ban it, and the United States prohibits the use of federal funds for either reproductive or therapeutic cloning.
The new study was funded by a foundation and the South Korean government.
If confirmed by other labs, it could prove significant because many illnesses that might one day be treated with stem cells, such as heart failure and vision loss, primarily affect adults. Patient-specific stem cells would have to be created from older cells, not infant or fetal ones. That now looks possible, though far from easy: Out of 39 tries, the scientists created stem cells only once for each donor.
Outside experts had different views of the study, which was led by Young Gie Chung of the Research Institute for Stem Cell Research at CHA Health Systems in Los Angeles.
Stem cell biologist George Daley of the Harvard Stem Cell Institute called it "an incremental advance" and "not earth-shattering."
Reproductive biologist Shoukhrat Mitalipov of Oregon Health and Science University, who developed the technique the CHA team adapted, was more positive. "The advance here is showing that (nuclear transfer) looks like it will work with people of all ages," he said in an interview.
A year ago, Mitalipov led the team that used nuclear transfer of fetal and infant DNA to produce stem cells, the first time that had been accomplished in humans of any age.
ELECTRIC JOLT
In therapeutic cloning, scientists use a zap of electricity to fuse a grown cell, usually a skin cell, with an ovum whose own DNA has been removed. The egg divides and multiplies, and within five or six days it develops into an embryo shaped like a hollow sphere.
The interior cells are "pluripotent" stem cells, which have the potential to develop into any kind of human cell.
If the embryo were implanted in a uterus, it could develop into a clone of the DNA donor, which is how Dolly was created. "Without regulations in place, such embryos could also be used for human reproductive cloning, although this would be unsafe and grossly unethical," said Dr Robert Lanza, chief scientist of Massachusetts-based biotech Advanced Cell Technology and a co-author of the new study.
The goal is to grow these embryonic stem cells in lab dishes and coax them to turn into specialized cells for therapeutic use against an illness the DNA donor has, such as Parkinson's disease, heart disease, multiple sclerosis or type-1 diabetes. Because the cells are genetically identical to the donor's, they would not be rejected by the immune system.
Despite more than 15 years of trying, scientists' single success at producing human stem cells through this cloning technique came a year ago. Mitalipov's team at Oregon had fused fetal and infant cells with donated eggs whose DNA had been removed and got them to develop into about 150-cell embryos.
One key to Mitalipov's success was letting the engineered eggs rest for 30 minutes before zapping them to start dividing.
Chung and his colleagues waited two hours before triggering the egg to start dividing, which Lanza believes was a key to their success: "It gives you time for the massive amount of genetic reprogramming required" to turn back the calendar on adult DNA so that it can direct the development of an embryo, he said in an interview.
It worked: They generated two healthy embryos, one from each adult donor, aged 35 and 75.
If each stem-cell line has to be created from scratch for each patient, the low success and expected high costs means that "only a few wealthy old men could do it," said Lanza. A big barrier to producing patient-specific stem-cell lines for tens of millions of people this way is that few women want to donate eggs, a sometimes painful process.
But it may not be necessary to make a unique cell line for each patient. Many people have genetically similar immune systems, Lanza said, so just "100 human embryonic stem cell lines would generate a complete match for over half the (U.S.) population," he said.
(Source: Reuters)




