
The Hanoi Medical University has started the first phase of clinical trials of COVID-19 vaccine ARCT-154 with the participation of 100 volunteers.
The Hanoi Medical University has started the first phase of clinical trials of COVID-19 vaccine ARCT-154 with the participation of 100 volunteers.
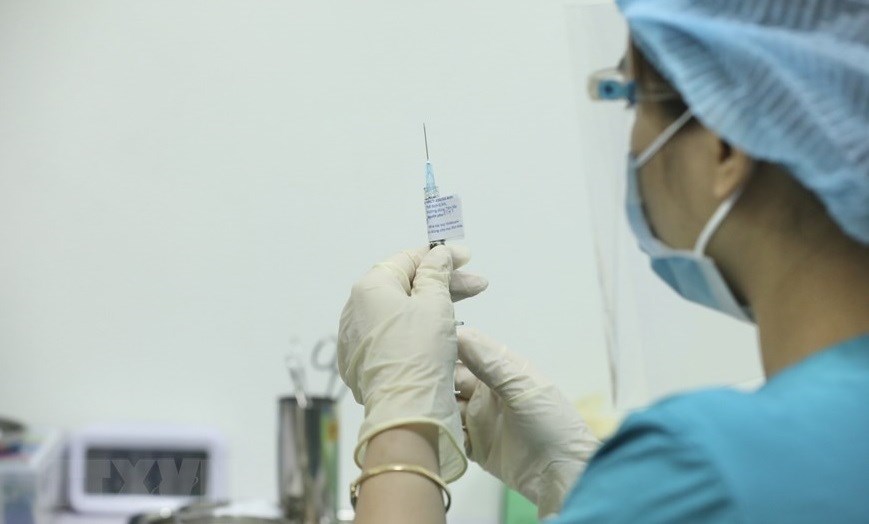 |
| The Hanoi Medical University starts the first phase of clinical trials of COVID-19 vaccine ARCT-154, a self-amplifying mRNA vaccine, with the participation of 100 volunteers from Hanoi. |
 |
| ARCT-154, a mRNA vaccine developed by US company Arcturus Therapeutics, Inc, which transferred the technology to a company in Vietnam. |
 |
| ARCT-154 is a self-amplifying mRNA vaccine designed to act against coronavirus variants, including the Delta. |
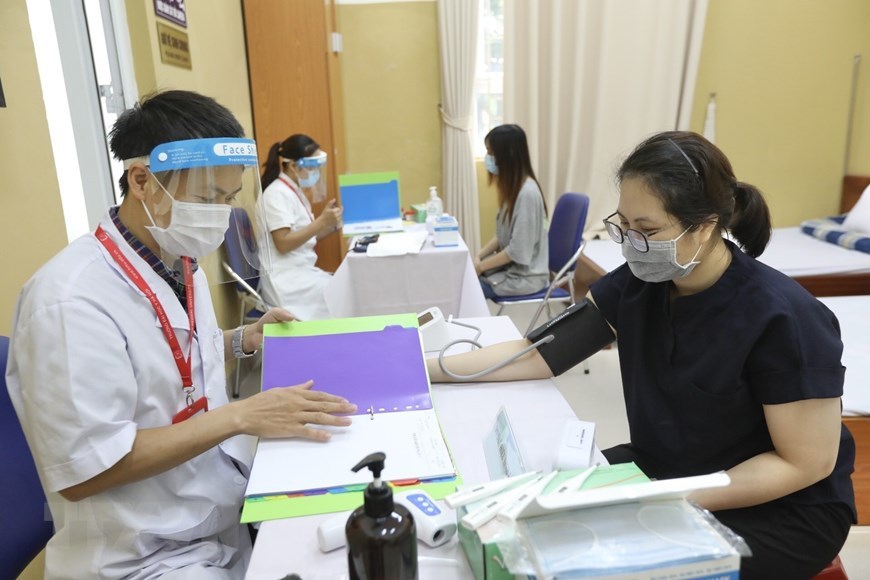 |
| ARCT-154 is the third COVID-19 vaccine put into human clinical trials in Vietnam and the first developed with mRNA technology. |
 |
| The clinical trials will be carried out in three phases with the participation of 21,000 volunteers, including 100 in phase one, 300 in phase two and 20,600 in phase three. |
 |
| Deputy Minister of Health Tran Van Thuan encourages volunteers before vaccination. |
(Source: VNA)





